Background
Primary CNS lymphoma (PCNSL) is a rare non-Hodgkin lymphoma that is often associated with immunosuppressed states. The Epstein-Barr Virus (EBV) may play a role in tumor pathogenicity in some cases. The objective of this study was to examine the patient characteristics, tumor pathology, and survival outcomes associated with EBV tumor status in patients with PCNSL.
Methods
This was a retrospective subset analysis from 17 academic medical centers that included 439 patients of ages 60 years and above with PCNSL (David K et al. ASH 2020). The associations between EBV status and clinical or demographical variables were tested by Fisher's exact test, Wilcoxon rank-sum test, or CMH trend test. Kaplan-Meier estimator was used to estimate survival probability. Survival difference between groups was tested by log-rank test for statistical significance. Confidence interval of survival rate was calculated using Greenwood's formula.
Results
A total of 247 patients with available EBV status were included in this analysis. Median age was 71 (range 60-84) and 44.5% were male. Notably, none of the patients were HIV-positive. Twenty-five patients (10.1%) had EBV positive tumors as detected by EBER (EBV-encoded RNA) in-situ hybridization or LMP1 immunohistochemistry (IHC), 17 of which were solid organ transplant (SOT)-related post-transplant lymphoproliferative disorders (PTLD) and 8 of which were not PTLD. All EBV-positive non-PTLDs were diffuse large B-cell lymphoma. Three (15%) SOT-related PTLDs were EBV-negative.
Patient characteristics analyzed included age at diagnosis, sex, ECOG performance status, history of prior or concurrent malignancies, history of solid organ transplant or autoimmune disease, history of allogeneic stem cell transplant, and immunosuppressive treatment. Of these, only a history of solid organ transplant or autoimmune disease (P<0.0001) and immunosuppressive treatment (P<0.0001) were highly correlated with EBV-positivity; there were no other patient factors associated with EBV status. For patients with EBV-positive SOT-related PTLD, the most common immunosuppressive medications were mycophenolate (n=13), calcineurin inhibitors (n=11), and prednisone (n=9). Only 1 of the EBV-positive non-PTLD cases had a history of autoimmune disease and was on mycophenolate and hydroxychloroquine.
Tumor characteristics analyzed included expression of C-MYC, BCL2, CD5, cell of origin markers (BCL6, MUM1, CD10), and CD20 through IHC, C-MYC and BCL2 translocation through FISH, histology, and involvement of brain parenchyma, CSF, spinal cord, and eyes. EBV-positive tumors were associated with low C-MYC (p=0.047) and BCL6 (p=0.0006) expression on immunohistochemistry, but not other factors. There were no significant differences in tumor characteristics between those with EBV-positive PTLD and EBV-positive non-PTLD.
Among patients with PTLD, 30% (n=6) did not receive primary chemotherapy, and the most common treatment regimens were high-dose methotrexate (HD-MTX) with or without rituximab (n=5) and rituximab alone (n=3). However, there was no significant difference in outcomes among PTLD patients who received chemotherapy or those who did not. Among EBV-positive non-PTLD patients, only 12.5% (n=1) did not receive primary chemotherapy and the most common treatment regimens were methotrexate/rituximab/temozolomide (n=3) and HD-MTX with or without rituximab (n=3).
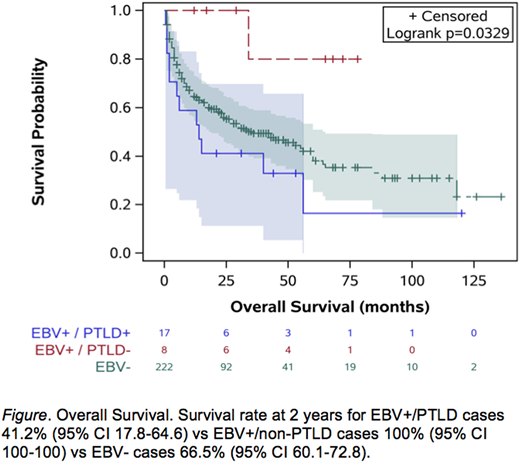
There was no difference in overall or progression free survival between patients with EBV-positive and EBV-negative tumors, or in outcomes among SOT-related PTLD patients regardless of EBV status. However, patients with EBV-positive non-PTLD PCNSL had better overall survival compared to patients with EBV-positive PTLD and EBV-negative tumors (p=0.033, Figure).
Conclusions
In this large observational study of older patients with PCNSL, the incidence of EBV positive tumors was overall low and was most commonly associated with SOT-related PTLD. Mycophenolate mofetil was the most common immunosuppressive medication. In those without PTLD, there were no patient or tumor factors that were associated with EBV status. Unexpectedly, non-PTLD EBV-positive PCNSL had superior outcomes to EBV-positive PTLD and EBV-negative PCNSL. Future studies of EBV-positive non-PTLDs are warranted to further evaluate the potential impact of EBV latency and the immune response on the tumor microenvironment.
Reddy:BMS: Consultancy, Research Funding; Celgene: Consultancy; Abbvie: Consultancy; Genentech: Research Funding; KITE Pharma: Consultancy. Bachanova:Karyopharma: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; FATE: Research Funding; Incyte: Research Funding; Gamida Cell: Membership on an entity's Board of Directors or advisory committees, Research Funding. Bond:Seattle Genetics: Honoraria. Goldlust:Tocagen: Membership on an entity's Board of Directors or advisory committees, Other: travel; BMS: Membership on an entity's Board of Directors or advisory committees, Other: travel; WEX: Consultancy, Other: travel; Cortice Bio: Consultancy, Other: travel; Boston Biomedical: Consultancy; COTA: Other; Novocure: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: travel, Research Funding, Speakers Bureau. Spurgeon:Gilead: Research Funding; Genentech: Research Funding; Cardinal Health: Honoraria; Bristol-Myers Squibb: Research Funding; VelosBio: Consultancy, Research Funding; Acerta: Research Funding; Janssen: Consultancy, Research Funding; AstraZeneca: Research Funding; Pharmacyclics: Consultancy; Beigene: Research Funding; Verastem: Research Funding; Genmab: Research Funding. Epperla:Verastem Oncology: Speakers Bureau; Pharmacyclics: Honoraria. Karmali:AstraZeneca: Speakers Bureau; Karyopharm: Honoraria; Takeda: Research Funding; BeiGene: Speakers Bureau; Gilead/Kite: Honoraria, Other, Research Funding, Speakers Bureau; BMS/Celgene/Juno: Honoraria, Other, Research Funding, Speakers Bureau. Naik:Celgene: Other: advisory board; Sanofi: Other: advisory board. Smith:Janssen: Consultancy; Celgene: Consultancy, Research Funding; Karyopharm: Consultancy, Research Funding; BMS: Consultancy; FortySeven: Research Funding; Pharmacyclics: Research Funding; Acerta: Research Funding; Genentech/Roche: Consultancy, Other: Support of parent study and funding of editorial support, Research Funding; TG Therapeutics: Consultancy, Research Funding. Rubenstein:Kymera: Research Funding. Kahl:BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca Pharmaceuticals LP: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Consultancy; AbbVie: Consultancy; Genentech: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Acerta: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche Laboratories Inc: Consultancy; Pharmacyclics LLC: Consultancy. Evens:Abbvie: Consultancy, Honoraria; Research To Practice: Honoraria, Speakers Bureau; MorphoSys: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Merck: Consultancy, Honoraria, Research Funding; Epizyme: Consultancy, Honoraria, Research Funding; Mylteni: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria, Research Funding. Martin:Kite: Consultancy; Morphosys: Consultancy; Regeneron: Consultancy; Incyte: Consultancy; Cellectar: Consultancy; Beigene: Consultancy; Bayer: Consultancy; I-MAB: Consultancy; Sandoz: Consultancy; Janssen: Consultancy; Karyopharm: Consultancy, Research Funding; Teneobio: Consultancy; Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal